what two amino acids are least likely to form an alpha helix

3-dimensional structure of an alpha helix in the protein crambin
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located iv residues earlier forth the protein sequence.
The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The proper noun 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bail.
Amidst types of local structure in proteins, the α-helix is the most extreme and the about predictable from sequence, as well as the most prevalent.

Discovery [edit]

Side view of an α-helix of alanine residues in atomic detail. 2 hydrogen bonds for the aforementioned peptide group are highlighted in magenta; the H to O distance is about ii Å (0.20 nm). The protein chain runs upward here; that is, its N-terminus is at the lesser and its C-terminus at the top. Note that the sidechains (blackness stubs) bending slightly downward, toward the N-terminus, while the peptide oxygens (red) point up and the peptide NHs (blue with grey stubs) indicate down.

Pinnacle view of the same helix shown above. Four carbonyl groups are pointing up toward the viewer, spaced roughly 100° apart on the circle, corresponding to 3.vi amino-acid residues per turn of the helix.
In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a feature repeat of ≈five.1 ångströms (0.51 nanometres).
Astbury initially proposed a linked-chain construction for the fibers. He later joined other researchers (notably the American chemist Maurice Huggins) in proposing that:
- the unstretched protein molecules formed a helix (which he called the α-form)
- the stretching caused the helix to uncoil, forming an extended land (which he chosen the β-form).
Although incorrect in their details, Astbury's models of these forms were correct in essence and correspond to modern elements of secondary structure, the α-helix and the β-strand (Astbury'southward classification was kept), which were adult by Linus Pauling, Robert Corey and Herman Branson in 1951 (meet below); that paper showed both right- and left-handed helices, although in 1960 the crystal construction of myoglobin[one] showed that the correct-handed class is the common one. Hans Neurath was the first to show that Astbury's models could not be correct in detail, because they involved clashes of atoms.[2] Neurath's paper and Astbury'due south data inspired H. S. Taylor,[3] Maurice Huggins[four] and Bragg and collaborators[five] to advise models of keratin that somewhat resemble the modern α-helix.
Two central developments in the modeling of the modern α-helix were: the right bond geometry, cheers to the crystal structure determinations of amino acids and peptides and Pauling's prediction of planar peptide bonds; and his relinquishing of the supposition of an integral number of residues per turn of the helix. The pivotal moment came in the early on spring of 1948, when Pauling defenseless a cold and went to bed. Being bored, he drew a polypeptide chain of roughly correct dimensions on a strip of newspaper and folded it into a helix, existence conscientious to maintain the planar peptide bonds. After a few attempts, he produced a model with physically plausible hydrogen bonds. Pauling then worked with Corey and Branson to confirm his model before publication.[vi] In 1954, Pauling was awarded his first Nobel Prize "for his research into the nature of the chemic bond and its awarding to the elucidation of the structure of complex substances"[7] (such as proteins), prominently including the structure of the α-helix.
Structure [edit]
Geometry and hydrogen bonding [edit]
The amino acids in an α-helix are arranged in a right-handed helical construction where each amino acid residue corresponds to a 100° plow in the helix (i.eastward., the helix has 3.vi residues per plow), and a translation of i.five Å (0.xv nm) forth the helical axis. Dunitz[eight] describes how Pauling's first commodity on the theme in fact shows a left-handed helix, the enantiomer of the truthful construction. Brusk pieces of left-handed helix sometimes occur with a large content of achiral glycine amino acids, but are unfavorable for the other normal, biological L-amino acids. The pitch of the blastoff-helix (the vertical distance betwixt consecutive turns of the helix) is 5.four Å (0.54 nm), which is the product of one.5 and 3.six. What is most important is that the N-H grouping of an amino acrid forms a hydrogen bail with the C=O group of the amino acrid four residues earlier; this repeated i + 4 → i hydrogen bonding is the most prominent characteristic of an α-helix. Official international nomenclature[nine] [ten] specifies two means of defining α-helices, rule 6.2 in terms of repeating φ, ψ torsion angles (see beneath) and rule 6.3 in terms of the combined design of pitch and hydrogen bonding. The α-helices tin be identified in protein construction using several computational methods, one of which is DSSP (Define Secondary Structure of Protein).[11]
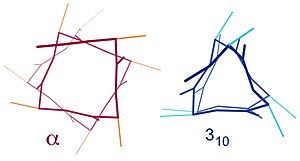
Contrast of helix stop views between α (offset squarish) vs 310 (triangular)
Similar structures include the three10 helix (i + 3 → i hydrogen bonding) and the π-helix (i + 5 → i hydrogen bonding). The α-helix can be described as a 3.613 helix, since the i + 4 spacing adds iii more than atoms to the H-bonded loop compared to the tighter 3x helix, and on average, 3.half dozen amino acids are involved in one ring of α-helix. The subscripts refer to the number of atoms (including the hydrogen) in the closed loop formed by the hydrogen bond.[12]

Ramachandran plot (φ,ψ plot), with data points for α-helical residues forming a dumbo diagonal cluster below and left of center, around the global energy minimum for backbone conformation.[13]
Residues in α-helices typically prefer backbone (φ,ψ) dihedral angles around (−60°, −45°), as shown in the image at right. In more than full general terms, they adopt dihedral angles such that the ψ dihedral bending of 1 balance and the φ dihedral angle of the adjacent residuum sum to roughly −105°. As a consequence, α-helical dihedral angles, in general, fall on a diagonal stripe on the Ramachandran diagram (of slope −1), ranging from (−90°, −fifteen°) to (−35°, −lxx°). For comparison, the sum of the dihedral angles for a three10 helix is roughly −75°, whereas that for the π-helix is roughly −130°. The general formula for the rotation angle Ω per residue of whatsoever polypeptide helix with trans isomers is given past the equation[14] [15]
- 3 cos Ω = i − four cos2 φ + ψ / 2
The α-helix is tightly packed; there is well-nigh no free space within the helix. The amino-acid side-chains are on the outside of the helix, and point roughly "downward" (i.eastward., toward the Northward-terminus), like the branches of an evergreen tree (Christmas tree effect). This directionality is sometimes used in preliminary, low-resolution electron-density maps to determine the direction of the protein backbone.[xvi]
Stability [edit]
Helices observed in proteins can range from 4 to over forty residues long, but a typical helix contains about ten amino acids (nearly 3 turns). In full general, short polypeptides do not showroom much α-helical structure in solution, since the entropic cost associated with the folding of the polypeptide concatenation is not compensated for by a sufficient amount of stabilizing interactions. In general, the backbone hydrogen bonds of α-helices are considered slightly weaker than those constitute in β-sheets, and are readily attacked past the ambient water molecules. All the same, in more hydrophobic environments such as the plasma membrane, or in the presence of co-solvents such as trifluoroethanol (TFE), or isolated from solvent in the gas phase,[17] oligopeptides readily adopt stable α-helical structure. Furthermore, crosslinks can exist incorporated into peptides to conformationally stabilize helical folds. Crosslinks stabilize the helical country past entropically destabilizing the unfolded state and past removing enthalpically stabilized "decoy" folds that compete with the fully helical state.[18] It has been shown that α-helices are more than stable, robust to mutations and designable than β-strands in natural proteins,[nineteen] and likewise in artificial designed proteins.[xx]

An α-helix in ultrahigh-resolution electron density contours, with oxygen atoms in cerise, nitrogen atoms in blueish, and hydrogen bonds as greenish dotted lines (PDB file 2NRL, 17–32). The Northward-terminus is at the top, here.
Visualization [edit]
The 3 almost popular ways of visualizing the blastoff-helical secondary structure of oligopeptide sequences are (i) a helical wheel,[21] (2) a wenxiang diagram,[22] and (3) a helical internet.[23] Each of these can be visualized with diverse software packages and spider web servers. To generate a modest number of diagrams, Heliquest[24] tin can exist used for helical wheels, and NetWheels[25] can be used for helical wheels and helical nets. To programmatically generate a large number of diagrams, helixvis[26] [27] can be used to draw helical wheels and wenxiang diagrams in the R and Python programming languages.
Experimental determination [edit]
Since the α-helix is defined by its hydrogen bonds and backbone conformation, the most detailed experimental evidence for α-helical structure comes from atomic-resolution 10-ray crystallography such equally the example shown at right. It is clear that all the backbone carbonyl oxygens bespeak down (toward the C-terminus) but splay out slightly, and the H-bonds are approximately parallel to the helix centrality. Protein structures from NMR spectroscopy as well testify helices well, with feature observations of nuclear Overhauser consequence (NOE) couplings between atoms on adjacent helical turns. In some cases, the private hydrogen bonds tin be observed directly equally a modest scalar coupling in NMR.
There are several lower-resolution methods for assigning general helical construction. The NMR chemic shifts (in particular of the Cα, Cβ and C′) and residual dipolar couplings are often feature of helices. The far-UV (170–250 nm) round dichroism spectrum of helices is also idiosyncratic, exhibiting a pronounced double minimum at around 208 and 222 nm. Infrared spectroscopy is rarely used, since the α-helical spectrum resembles that of a random roll (although these might exist discerned past, eastward.thou., hydrogen-deuterium exchange). Finally, cryo electron microscopy is now capable of discerning individual α-helices within a protein, although their assignment to residues is still an active area of enquiry.
Long homopolymers of amino acids often form helices if soluble. Such long, isolated helices tin also be detected past other methods, such as dielectric relaxation, menstruum birefringence, and measurements of the improvidence constant. In stricter terms, these methods notice only the characteristic prolate (long cigar-like) hydrodynamic shape of a helix, or its big dipole moment.
Amino-acid propensities [edit]
Different amino-acid sequences have different propensities for forming α-helical construction. Methionine, alanine, leucine, glutamate, and lysine uncharged ("MALEK" in the amino-acid 1-letter of the alphabet codes) all take especially loftier helix-forming propensities, whereas proline and glycine have poor helix-forming propensities.[28] Proline either breaks or kinks a helix, both considering information technology cannot donate an amide hydrogen bond (having no amide hydrogen), and too because its sidechain interferes sterically with the backbone of the preceding turn – inside a helix, this forces a bend of well-nigh 30° in the helix's centrality.[12] All the same, proline is ofttimes seen as the first residue of a helix, it is presumed due to its structural rigidity. At the other extreme, glycine also tends to disrupt helices because its high conformational flexibility makes it entropically expensive to adopt the relatively constrained α-helical structure.
Table of standard amino acid alpha-helical propensities [edit]
Estimated differences in free energy, Δ(ΔYard), estimated in kcal/mol per balance in an α-helical configuration, relative to alanine arbitrarily set up as naught. College numbers (more positive free energies) are less favoured. Significant deviations from these average numbers are possible, depending on the identities of the neighbouring residues.
-
Differences in free energy per residue[29] Amino acid 3-
letter1-
letter of the alphabetHelical penalty kcal/mol kJ/mol Alanine Ala A 0.00 0.00 Arginine Arg R 0.21 0.88 Asparagine Asn N 0.65 2.72 Aspartic acid Asp D 0.69 ii.89 Cysteine Cys C 0.68 2.85 Glutamic acrid Glu E 0.forty 1.67 Glutamine Gln Q 0.39 1.63 Glycine Gly G one.00 4.18 Histidine His H 0.61 ii.55 Isoleucine Ile I 0.41 one.72 Leucine Leu L 0.21 0.88 Lysine Lys G 0.26 1.09 Methionine Met M 0.24 1.00 Phenylalanine Phe F 0.54 two.26 Proline Pro P 3.xvi 13.22 Serine Ser S 0.50 2.09 Threonine Thr T 0.66 2.76 Tryptophan Trp Due west 0.49 2.05 Tyrosine Tyr Y 0.53 2.22 Valine Val V 0.61 2.55
Dipole moment [edit]
A helix has an overall dipole moment due to the amass effect of the individual microdipoles from the carbonyl groups of the peptide bond pointing along the helix axis.[30] The effects of this macrodipole are a matter of some controversy. α-helices often occur with the N-terminal end jump by a negatively charged grouping, sometimes an amino acid side chain such as glutamate or aspartate, or sometimes a phosphate ion. Some regard the helix macrodipole as interacting electrostatically with such groups. Others feel that this is misleading and information technology is more than realistic to say that the hydrogen bond potential of the free NH groups at the N-terminus of an α-helix can be satisfied by hydrogen bonding; this can also be regarded as set of interactions between local microdipoles such as C=O···H−N.[31] [32]
Coiled coils [edit]
Coiled-coil α helices are highly stable forms in which two or more than helices wrap around each other in a "supercoil" construction. Coiled coils comprise a highly feature sequence motif known as a heptad echo, in which the motif repeats itself every seven residues along the sequence (amino acid residues, not Dna base-pairs). The first and peculiarly the fourth residues (known as the a and d positions) are most always hydrophobic; the fourth balance is typically leucine – this gives ascent to the name of the structural motif chosen a leucine attachment, which is a type of coiled-curlicue. These hydrophobic residues pack together in the interior of the helix bundle. In general, the fifth and seventh residues (the eastward and g positions) have opposing charges and form a salt bridge stabilized by electrostatic interactions. Gristly proteins such every bit keratin or the "stalks" of myosin or kinesin often adopt coiled-coil structures, as do several dimerizing proteins. A pair of coiled-coils – a four-helix bundle – is a very common structural motif in proteins. For example, information technology occurs in homo growth hormone and several varieties of cytochrome. The Rop poly peptide, which promotes plasmid replication in bacteria, is an interesting case in which a single polypeptide forms a coiled-gyre and 2 monomers assemble to class a four-helix package.
Facial arrangements [edit]
The amino acids that make up a particular helix tin can exist plotted on a helical bicycle, a representation that illustrates the orientations of the constituent amino acids (run into the commodity for leucine zipper for such a diagram). Oft in globular proteins, equally well equally in specialized structures such every bit coiled-coils and leucine zippers, an α-helix volition showroom two "faces" – 1 containing predominantly hydrophobic amino acids oriented toward the interior of the protein, in the hydrophobic core, and one containing predominantly polar amino acids oriented toward the solvent-exposed surface of the protein.
Changes in bounden orientation also occur for facially-organized oligopeptides. This pattern is especially common in antimicrobial peptides, and many models have been devised to draw how this relates to their function. Common to many of them is that the hydrophobic face of the antimicrobial peptide forms pores in the plasma membrane after associating with the fat chains at the membrane core.[33] [34]
Larger-scale assemblies [edit]

The Hemoglobin molecule has 4 heme-binding subunits, each made largely of α-helices.
Myoglobin and hemoglobin, the start two proteins whose structures were solved past 10-ray crystallography, have very similar folds made up of most seventy% α-helix, with the rest beingness non-repetitive regions, or "loops" that connect the helices. In classifying proteins by their dominant fold, the Structural Classification of Proteins database maintains a large category specifically for all-α proteins.
Hemoglobin then has an fifty-fifty larger-scale quaternary construction, in which the functional oxygen-binding molecule is made up of iv subunits.
Functional roles [edit]


Bovine rhodopsin (PDB file 1GZM), with a bundle of vii helices crossing the membrane (membrane surfaces marked by horizontal lines)
DNA binding [edit]
α-Helices have particular significance in Dna binding motifs, including helix-turn-helix motifs, leucine zipper motifs and zinc finger motifs. This is because of the convenient structural fact that the diameter of an α-helix is about 12 Å (1.ii nm) including an boilerplate set of sidechains, about the same as the width of the major groove in B-form Deoxyribonucleic acid, and also because coiled-coil (or leucine zipper) dimers of helices can readily position a pair of interaction surfaces to contact the sort of symmetrical repeat common in double-helical DNA.[35] An example of both aspects is the transcription factor Max (run into paradigm at left), which uses a helical coiled whorl to dimerize, positioning another pair of helices for interaction in ii successive turns of the Dna major groove.
Membrane spanning [edit]
α-Helices are also the virtually common protein structure element that crosses biological membranes (transmembrane protein),[36] it is presumed because the helical structure can satisfy all backbone hydrogen-bonds internally, leaving no polar groups exposed to the membrane if the sidechains are hydrophobic. Proteins are sometimes anchored by a single membrane-spanning helix, sometimes by a pair, and sometimes by a helix bundle, well-nigh classically consisting of seven helices arranged up-and-down in a ring such every bit for rhodopsins (meet image at correct) or for G protein–coupled receptors (GPCRs). The structural stability between pairs of α-Helical transmembrane domains rely on conserved membrane interhelical packing motifs, for example, the Glycine-xxx-Glycine (or modest-xxx-pocket-sized) motif.[37]
Mechanical backdrop [edit]
α-Helices nether axial tensile deformation, a characteristic loading status that appears in many alpha-helix-rich filaments and tissues, results in a feature 3-phase behavior of stiff-soft-potent tangent modulus.[38] Phase I corresponds to the small-deformation government during which the helix is stretched homogeneously, followed past stage II, in which alpha-helical turns intermission mediated by the rupture of groups of H-bonds. Phase 3 is typically associated with big-deformation covalent bond stretching.
Dynamical features [edit]
Blastoff-helices in proteins may have depression-frequency piano accordion-like motion as observed past the Raman spectroscopy[39] and analyzed via the quasi-continuum model.[40] [41] Helices not stabilized by tertiary interactions evidence dynamic behavior, which can exist mainly attributed to helix fraying from the ends.[42]
Helix–coil transition [edit]
Homopolymers of amino acids (such as polylysine) can adopt α-helical structure at low temperature that is "melted out" at high temperatures. This helix–gyre transition was one time thought to be analogous to protein denaturation. The statistical mechanics of this transition tin be modeled using an elegant transfer matrix method, characterized by 2 parameters: the propensity to initiate a helix and the propensity to extend a helix.
In art [edit]

At least five artists accept made explicit reference to the α-helix in their work: Julie Newdoll in painting and Julian Voss-Andreae, Bathsheba Grossman, Byron Rubin, and Mike Tyka in sculpture.
San Francisco area artist Julie Newdoll,[43] who holds a degree in Microbiology with a small in art, has specialized in paintings inspired by microscopic images and molecules since 1990. Her painting "Rise of the Alpha Helix" (2003) features human figures arranged in an α helical arrangement. According to the artist, "the flowers reverberate the diverse types of sidechains that each amino acid holds out to the world".[43] This same metaphor is also echoed from the scientist'southward side: "β sheets do not prove a stiff repetitious regularity but menstruum in svelte, twisting curves, and even the α-helix is regular more in the manner of a flower stalk, whose branching nodes show the influence of surroundings, developmental history, and the evolution of each function to match its own idiosyncratic function."[12]
Julian Voss-Andreae is a German-born sculptor with degrees in experimental physics and sculpture. Since 2001 Voss-Andreae creates "protein sculptures"[44] based on poly peptide structure with the α-helix beingness one of his preferred objects. Voss-Andreae has fabricated α-helix sculptures from various materials including bamboo and whole trees. A monument Voss-Andreae created in 2004 to gloat the memory of Linus Pauling, the discoverer of the α-helix, is fashioned from a large steel beam rearranged in the structure of the α-helix. The 10-foot-tall (3 1000), bright-red sculpture stands in front of Pauling'southward childhood dwelling in Portland, Oregon.
Ribbon diagrams of α-helices are a prominent element in the laser-etched crystal sculptures of protein structures created by artist Bathsheba Grossman, such as those of insulin, hemoglobin, and DNA polymerase.[45] Byron Rubin is a former protein crystallographer now professional person sculptor in metal of proteins, nucleic acids, and drug molecules – many of which featuring α-helices, such as subtilisin, human growth hormone, and phospholipase A2.[46]
Mike Tyka is a computational biochemist at the University of Washington working with David Bakery. Tyka has been making sculptures of protein molecules since 2010 from copper and steel, including ubiquitin and a potassium channel tetramer.[47]
Meet also [edit]
- 3x helix
- Beta sheet
- Davydov soliton
- Folding (chemical science)
- Knobs into holes packing
- Pi helix
- Proteopedia Helices_in_Proteins
References [edit]
- ^ Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR, Phillips DC, Shore VC (Feb 1960). "Structure of myoglobin: A three-dimensional Fourier synthesis at ii Å resolution". Nature. 185 (4711): 422–vii. Bibcode:1960Natur.185..422K. doi:10.1038/185422a0. PMID 18990802. S2CID 4167651.
- ^ Neurath H (1940). "Intramolecular folding of polypeptide chains in relation to protein structure". Journal of Concrete Chemistry. 44 (3): 296–305. doi:10.1021/j150399a003.
- ^ Taylor HS (1942). "Large molecules through atomic glasses". Proceedings of the American Philosophical Order. 85 (1): one–12. JSTOR 985121.
- ^ Huggins M (1943). "The structure of gristly proteins". Chemical Reviews. 32 (2): 195–218. doi:10.1021/cr60102a002.
- ^ Bragg WL, Kendrew JC, Perutz MF (1950). "Polypeptide concatenation configurations in crystalline proteins". Proceedings of the Royal Guild of London. Series A. Mathematical and Physical Sciences. 203 (1074): 321–?. Bibcode:1950RSPSA.203..321B. doi:x.1098/rspa.1950.0142. S2CID 93804323.
- ^ Pauling Fifty, Corey RB, Branson 60 minutes (Apr 1951). "The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain". Proceedings of the National Academy of Sciences of the United states of america of America. 37 (4): 205–xi. Bibcode:1951PNAS...37..205P. doi:10.1073/pnas.37.4.205. PMC1063337. PMID 14816373.
- ^ "The Nobel Prize in Chemistry 1954".
- ^ Dunitz J (2001). "Pauling'due south Left-Handed α-Helix". Angewandte Chemie International Edition. twoscore (22): 4167–4173. doi:10.1002/1521-3773(20011119)40:22<4167::AID-ANIE4167>3.0.CO;2-Q. PMID 29712120.
- ^ IUPAC-IUB Commission on Biochemical Nomenclature (1970). "Abbreviations and symbols for the clarification of the conformation of polypeptide chains". Journal of Biological Chemistry. 245 (24): 6489–6497. doi:10.1016/S0021-9258(eighteen)62561-X.
- ^ "Polypeptide Conformations one and 2". world wide web.sbcs.qmul.air-conditioning.uk . Retrieved 5 November 2018.
- ^ Kabsch W, Sander C (December 1983). "Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features". Biopolymers. 22 (12): 2577–637. doi:ten.1002/bip.360221211. PMID 6667333. S2CID 29185760.
- ^ a b c Richardson JS (1981). "The anatomy and taxonomy of protein structure". Advances in Protein Chemistry. 34: 167–339. doi:10.1016/S0065-3233(08)60520-3. ISBN9780120342341. PMID 7020376.
- ^ Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC (February 2003). "Structure validation past Calpha geometry: phi,psi and Cbeta departure". Proteins. 50 (three): 437–50. doi:10.1002/prot.10286. PMID 12557186. S2CID 8358424.
- ^ Dickerson RE, Geis I (1969), Structure and Activeness of Proteins, Harper, New York
- ^ Zorko, Matjaž (2010). "Structural Organisation of Proteins". In Langel, Ülo; Cravatt, Benjamin F.; Gräslund, Astrid; von Heijne, Gunnar; State, Tiit; Niessen, Sherry; Zorko, Matjaž (eds.). Introduction to Peptides and Proteins. Boca Raton: CRC Printing. pp. 36–57. ISBN9781439882047.
- ^ Terwilliger TC (March 2010). "Rapid model building of blastoff-helices in electron-density maps". Acta Crystallographica Department D. 66 (Pt 3): 268–75. doi:10.1107/S0907444910000314. PMC2827347. PMID 20179338.
- ^ Hudgins RR, Jarrold MF (1999). "Helix Formation in Unsolvated Alanine-Based Peptides: Helical Monomers and Helical Dimers". Periodical of the American Chemic Social club. 121 (xiv): 3494–3501. doi:10.1021/ja983996a.
- ^ Kutchukian PS, Yang JS, Verdine GL, Shakhnovich EI (April 2009). "All-cantlet model for stabilization of blastoff-helical structure in peptides by hydrocarbon staples". Journal of the American Chemical Society. 131 (xiii): 4622–vii. doi:ten.1021/ja805037p. PMC2735086. PMID 19334772.
- ^ Abrusan G, Marsh JA (2016). "Alpha helices are more than robust to mutations than beta strands". PLOS Computational Biology. 12 (12): e1005242. Bibcode:2016PLSCB..12E5242A. doi:10.1371/periodical.pcbi.1005242. PMC5147804. PMID 27935949.
- ^ Rocklin GJ, et al. (2017). "Global assay of protein folding using massively parallel design, synthesis, and testing". Science. 357 (6347): 168–175. Bibcode:2017Sci...357..168R. doi:10.1126/science.aan0693. PMC5568797. PMID 28706065.
- ^ Schiffer One thousand, Edmundson AB (1967). "Utilize of helical wheels to represent the structures of proteins and to place segments with helical potential". Biophysical Periodical. 7 (two): 121–135. doi:10.1016/S0006-3495(67)86579-2.
- ^ Chou KC, Zhang CT, Maggiora GM (1997). "Disposition of amphiphilic helices in heteropolar environments". Proteins: Structure, Role, and Genetics. 28: 99–108.
- ^ Dunnill P (1968). "The Utilise of Helical Internet-Diagrams to Stand for Protein Structures". Biophysical Journal. eight (7): 865–875. doi:10.1016/S0006-3495(68)86525-7. PMC1367563. PMID 5699810.
- ^ Gautier R, Douguet D, Antonny B, Drin Thou (2008). "HELIQUEST: a web server to screen sequences with specific blastoff-helical properties". Bioinformatics. 24 (18): 2101–2102. doi:10.1093/bioinformatics/btn392.
- ^ Mol AR, Castro MS, Fontes Due west (2018). "NetWheels: A web awarding to create high quality peptide helical bicycle and cyberspace projections". bioRxiv. doi:10.1101/416347.
- ^ Wadhwa RR, Subramanian Five, Stevens-Truss R (2018). "Visualizing alpha-helical peptides in R with helixvis". Journal of Open Source Software. 3 (31): 1008. doi:10.21105/joss.01008.
- ^ Subramanian Five, Wadhwa RR, Stevens-Truss R (2020). "Helixvis: Visualize alpha-helical peptides in Python". chemRxiv.
- ^ Pace CN, Scholtz JM (July 1998). "A helix propensity scale based on experimental studies of peptides and proteins". Biophysical Journal. 75 (ane): 422–7. Bibcode:1998BpJ....75..422N. doi:10.1016/S0006-3495(98)77529-0. PMC1299714. PMID 9649402.
- ^ Pace, C. Nick; Scholtz, J. Martin (1998). "A Helix Propensity Calibration Based on Experimental Studies of Peptides and Proteins". Biophysical Journal. Vol. 75. pp. 422–427. Bibcode:1998BpJ....75..422N. doi:10.1016/s0006-3495(98)77529-0.
- ^ Hol WG, van Duijnen PT, Berendsen HJ (1978). "The blastoff helix dipole and the properties of proteins". Nature. 273 (5662): 443–446. Bibcode:1978Natur.273..443H. doi:ten.1038/273443a0. PMID 661956. S2CID 4147335.
- ^ He JJ, Quiocho FA (October 1993). "Dominant role of local dipoles in stabilizing uncompensated charges on a sulfate sequestered in a periplasmic active transport poly peptide". Poly peptide Science. 2 (10): 1643–seven. doi:10.1002/pro.5560021010. PMC2142251. PMID 8251939.
- ^ Milner-White EJ (November 1997). "The partial accuse of the nitrogen atom in peptide bonds". Protein Science. 6 (11): 2477–82. doi:ten.1002/pro.5560061125. PMC2143592. PMID 9385654.
- ^ Kohn, Eric G.; Shirley, David J.; Arotsky, Lubov; Picciano, Angela M.; Ridgway, Zachary; Urban, Michael W.; Carone, Benjamin R.; Caputo, Gregory A. (2018-02-04). "Role of Cationic Side Chains in the Antimicrobial Activeness of C18G". Molecules. 23 (2): 329. doi:10.3390/molecules23020329. PMC6017431. PMID 29401708.
- ^ Toke, Orsolya (2005). "Antimicrobial peptides: new candidates in the fight confronting bacterial infections". Biopolymers. lxxx (six): 717–735. doi:10.1002/bip.20286. ISSN 0006-3525. PMID 15880793.
- ^ Branden & Tooze, affiliate 10
- ^ Branden & Tooze, chapter 12.
- ^ Nash A, Notman R, Dixon AM (2015). "De novo design of transmembrane helix–helix interactions and measurement of stability in a biological membrane". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1848 (five): 1248–57. doi:10.1016/j.bbamem.2015.02.020. PMID 25732028.
- ^ Ackbarow T, Chen X, Keten South, Buehler MJ (October 2007). "Hierarchies, multiple energy barriers, and robustness govern the fracture mechanics of alpha-helical and beta-sail poly peptide domains". Proceedings of the National University of Sciences of the United states of america of America. 104 (42): 16410–5. Bibcode:2007PNAS..10416410A. doi:ten.1073/pnas.0705759104. PMC2034213. PMID 17925444.
- ^ Painter PC, Mosher LE, Rhoads C (July 1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers. 21 (vii): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
- ^ Chou KC (December 1983). "Identification of depression-frequency modes in poly peptide molecules". The Biochemical Journal. 215 (3): 465–9. doi:x.1042/bj2150465. PMC1152424. PMID 6362659.
- ^ Chou KC (May 1984). "Biological functions of depression-frequency vibrations (phonons). III. Helical structures and microenvironment". Biophysical Periodical. 45 (v): 881–9. Bibcode:1984BpJ....45..881C. doi:ten.1016/S0006-3495(84)84234-4. PMC1434967. PMID 6428481.
- ^ Fierz B, Reiner A, Kiefhaber T (January 2009). "Local conformational dynamics in alpha-helices measured by fast triplet transfer". Proceedings of the National Academy of Sciences of the Us of America. 106 (iv): 1057–62. Bibcode:2009PNAS..106.1057F. doi:10.1073/pnas.0808581106. PMC2633579. PMID 19131517.
- ^ a b "Julie Newdoll Scientifically Inspired Art, Music, Lath Games". www.brushwithscience.com . Retrieved 2016-04-06 .
- ^ Voss-Andreae J (2005). "Poly peptide Sculptures: Life's Building Blocks Inspire Fine art". Leonardo. 38: 41–45. doi:10.1162/leon.2005.38.1.41. S2CID 57558522.
- ^ Grossman, Bathsheba. "About the Artist". Bathsheba Sculpture . Retrieved 2016-04-06 .
- ^ "Well-nigh". molecularsculpture.com . Retrieved 2016-04-06 .
- ^ Tyka, Mike. "Nigh". world wide web.miketyka.com . Retrieved 2016-04-06 .
Farther reading [edit]
- Tooze J, Brändén C (1999). Introduction to poly peptide structure. New York: Garland Pub. ISBN0-8153-2304-2. .
- Eisenberg D (September 2003). "The discovery of the alpha-helix and beta-sail, the principal structural features of proteins". Proceedings of the National Academy of Sciences of the U.s.a. of America. 100 (20): 11207–ten. Bibcode:2003PNAS..10011207E. doi:ten.1073/pnas.2034522100. PMC208735. PMID 12966187.
- Astbury WT, Woods HJ (1931). "The Molecular Weights of Proteins". Nature. 127 (3209): 663–665. Bibcode:1931Natur.127..663A. doi:x.1038/127663b0. S2CID 4133226.
- Astbury WT, Street A (1931). "X-ray studies of the structures of hair, wool and related fibres. I. General". Trans. R. Soc. Lond. A230: 75–101. Bibcode:1932RSPTA.230...75A. doi:10.1098/rsta.1932.0003.
- Astbury WT (1933). "Some Issues in the 10-ray Assay of the Structure of Brute Hairs and Other Protein Fibers". Trans. Faraday Soc. 29 (140): 193–211. doi:10.1039/tf9332900193.
- Astbury WT, Woods HJ (1934). "Ten-ray studies of the structures of hair, wool and related fibres. 2. The molecular structure and elastic backdrop of pilus keratin". Philosophical Transactions of the Royal Social club of London, Series A. 232 (707–720): 333–394. Bibcode:1934RSPTA.232..333A. doi:10.1098/rsta.1934.0010.
- Astbury WT, Sisson WA (1935). "X-ray studies of the structures of hair, wool and related fibres. III. The configuration of the keratin molecule and its orientation in the biological cell". Proceedings of the Imperial Society. A150 (871): 533–551. Bibcode:1935RSPSA.150..533A. doi:ten.1098/rspa.1935.0121.
- Sugeta H, Miyazawa T (1967). "General Method for Computing Helical Parameters of Polymer Bondage from Bond Lengths, Bond Angles, and Internal-Rotation Angles". Biopolymers. five (7): 673–679. doi:10.1002/bip.1967.360050708. S2CID 97785907.
- Wada A (1976). "The blastoff-helix as an electric macro-dipole". Advances in Biophysics: 1–63. PMID 797240.
- Chothia C, Levitt One thousand, Richardson D (Oct 1977). "Structure of proteins: packing of alpha-helices and pleated sheets". Proceedings of the National University of Sciences of the United States of America. 74 (10): 4130–iv. Bibcode:1977PNAS...74.4130C. doi:10.1073/pnas.74.10.4130. PMC431889. PMID 270659.
- Chothia C, Levitt M, Richardson D (Jan 1981). "Helix to helix packing in proteins". Journal of Molecular Biology. 145 (i): 215–l. doi:10.1016/0022-2836(81)90341-seven. PMID 7265198.
- Hol WG (1985). "The role of the blastoff-helix dipole in protein function and structure". Progress in Biophysics and Molecular Biology. 45 (3): 149–95. doi:x.1016/0079-6107(85)90001-X. PMID 3892583.
- Barlow DJ, Thornton JM (June 1988). "Helix geometry in proteins". Journal of Molecular Biology. 201 (3): 601–nineteen. doi:10.1016/0022-2836(88)90641-ix. PMID 3418712.
- Murzin AG, Finkelstein AV (December 1988). "General architecture of the alpha-helical globule". Journal of Molecular Biology. 204 (3): 749–69. doi:10.1016/0022-2836(88)90366-X. PMID 3225849.
External links [edit]
- NetSurfP ver. 1.1 – Protein Surface Accessibility and Secondary Construction Predictions
- α-helix rotational bending calculator
- Artist Julie Newdoll's website
- Artist Julian Voss-Andreae's website
Source: https://en.wikipedia.org/wiki/Alpha_helix
0 Response to "what two amino acids are least likely to form an alpha helix"
Postar um comentário